Software for calculation of salt precipitation
Free demo program
Software for calculation of salt precipitation based on the Extended UNIQUAC model can be performed with Microsoft Excel® as user interface. MATLAB, SysCAD or any other software that can connect to a Dynamic Link Library (DLL) can be used as interface too.
A digitally signed sample program can be downloaded free. The program includes the ions Na+, H+, Cl–, HSO4–, SO42-, and OH–. The sample program is only limited by the number of ions it includes. The types of calculations performed with the sample program are identical to the types of calculations that can be performed with extended versions of the program.
Based on user input of temperature and composition, the program performs the following calculations:
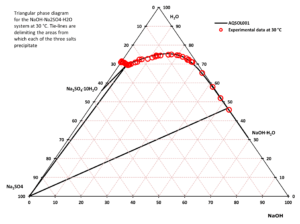
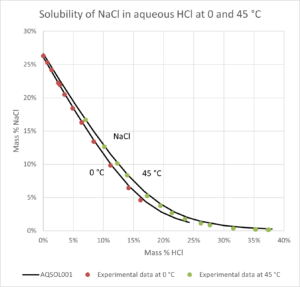
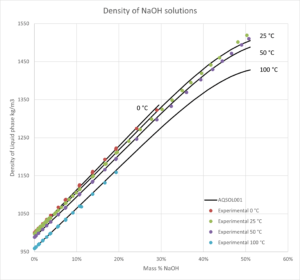
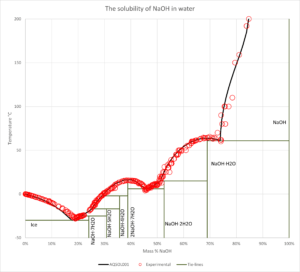
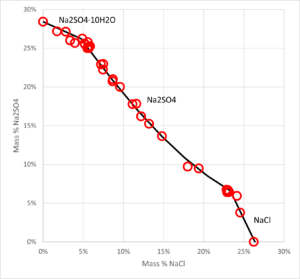
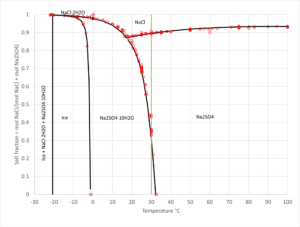
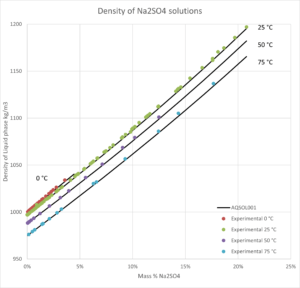
- Solid-liquid equilibrium, bubble point pressure, saturation index (Degree of saturation/supersaturation), enthalpy of formation, heat capacity, water activity, mean molal activity coefficient, density, freezing point depression, phase diagrams for salt systems
- The software can be used for calculating the amount and type of salts that will precipitate from a solution at a given temperature, simulation of fractional crystallization processes and much more.
The calculation accuracy achieved with this sample program is typical for the performance of the extended versions of the program. The license will expire after approximately one year. Use the “License Information” button in the “props” sheet to see what time the license expires. Then come back and download a new version. The sample program comes with a short manual, that explains about installation and use of the program.
Extended versions of the software
Pressure dependent solubility
An extended version of the program including the pressure dependency of solubility is available. This program contains the ions Na+, H+, Mg2+, Ca2+, Ba2+, Sr2+, Cl–, OH–, SO42-, HSO4–, CO32-, HCO3 and is meant for calculations of oil field scale or prediction of precipitation in geothermal energy production. More details about the oil field scaling calculation program here.
Integration with MATLAB from MathWorks®
The free demo program and extended versions of the program can be integrated with MATLAB. This requires a MATLAB header file which can be downloaded together with a small MATLAB script, and more information. Download header file and information about calling the model from MATLAB by clicking MATLAB_Information
Integration with SysCAD Plant Simulation Software
Our free demo program and extended versions of the program can be integrated with plant simulation software from SysCAD. SysCAD is a powerful and versatile plant simulation software, and can be used to simulate the simplest processing circuit through to a complex full plant operation. By integrating our software with SysCAD you can benefit the most from both software packages. Please contact SysCAD to purchase their software
Calculation of salt precipitation
A macro-enabled Microsoft Excel® file included in the download is used as interface to the free demo program. In the excel file you can perform your own calculations with the model. You will also see a number of calculation examples which have been calculated already. These are included in the gallery below: